Research
The Tata Institute for Genetics and Society's research initiatives leverage new technologies developed at UC San Diego based on self-propagating genetic elements we refer to as “Active Genetics."
Combating Vector-borne Disease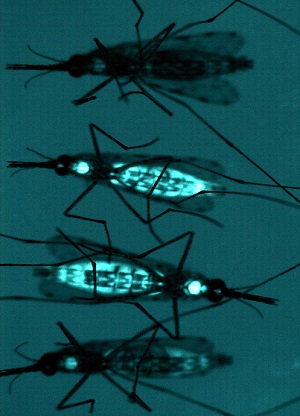
Pioneering experiments conducted at UC San Diego and UC Irvine have demonstrated that the malaria vector mosquito Anopheles stephensi can be genetically engineered using Active Genetics to express genes targeted against the malarial parasite Plasmodium falciparum, and that this new trait is inherited by nearly all of the mosquitoes’ progeny. Institute researchers and international collaborators are expanding on this work with a goal of developing mosquito strains that may ultimately be used to substantially reduce malaria transmission, using a vector replacement rather than a vector-elimination strategy. In addition to combating malaria, a disease that causes an estimated 450,000 worldwide deaths per year, this approach may also be leveraged against other mosquito-borne-disease agents, including Dengue, Chikungunya and Zika virus.
The Institute’s vector-borne disease program brings together international experts from molecular biology, entomology, public health, community engagement and regulation. Future field trials will adhere to guidelines developed by the World Health Organization, National Academies of Sciences and regulatory agencies of the United States and India. The Institute is following a phased approach to test both safety and efficacy of the strains as our work progresses.
Broader Applications
Several potentially impactful applications of Active Genetics are also being explored by a network of highly interactive faculty at UC San Diego who seek to develop this platform for use in other invertebrate species, vertebrates and plants. Applications include cell engineering, control of crop pests, development of new agricultural crop strains, creation of new humanized mouse models for studying and treating diseases such as cancer and potentially restoring microbial sensitivity to antibiotics.
TIGS and the COVID-19 Pandemic
In response to interest in the biology of COVID-19 infection, potential treatments, and anticipated vaccine development, a series of two UCTV (University of California Television) programs titled A Deep Look into the Biology and Evolution of COVID-19 and A Deep Look into COVID-19 Vaccines, Drugs, and the Evolutionary Arms Race were developed and published, with Professor Suresh Subramani moderating discussions with experts in the field of infectious disease.
Tata Chancellor’s Endowed Professor, Karthik Muralidharan, is participating in the policy outreach in the aftermath of COVID-19 with contributions to the following conversations, blogs and op-eds:
- The Road to Recovery: Rebuilding Governance and Finance in Indian States – India Leaders for Social Sector, 5th June, 2020
- A post-COVID-19 social protection architecture for India – Hindustan Times, 10th June, 2020
- Sound public health policy need of hour – Hindustan Times, 12th May, 2020
- A blueprint to finance higher public spending – Hindustan Times, 3rd May, 2020
TIGS Publications
Bier, E. and Sober, E. Gene Editing and the War Against Malaria. American Scientist 108, 162-169 (2020)
Buchman, A.; Gamez, S.; Li, M.; Antoshechkin, I.; Li, H.-H.; Wang, H.-W.; Chen, C.-H.; Klein, M. J.; Duchemin, J.-B.; Crowe, J.E., Jr; Paradkar, P.N.; Akbari, O.S. Broad dengue neutralization in mosquitoes expressing an engineered antibody. PLOS Pathogens 16 (1): e1008103 (2020) https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1008103
Del Amo, V.L.; Bishop, A.L.; Sanchez, H.M; Bennett, J.B.; Feng, X.; Marshall, J.M.; Bier. E; Gantz, V.M. A transcomplimenting gene drive provides a flexible platform for laboratory investigation and potential field deployment. Nature Communications 11, 352 (2020)
Pham, T.B.; Phong, C.H.; Bennett, J.B.; Hwang, K.; Jasinskiene, N.; Parker, K.; Stillinger, D.; Marshall, J.M.; Carballar-Lejarazú, R.; James, A.A. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genetics, 15(12):e1008440. PMID:31856182 (2019) https://doi.org/10.1371/journal.pgen.1008440
Valderrama, J.A.; Kulkarni, S.S.; Nizet, V.;Bier, E. A bacterial gene-drive system efficiently edits and inactivates a high copy number antibiotic resistance locus. Nature Communications 10, 5726 (2019)
Kandul, N.P; Liu, J.; Buchman, A.B.; Gantz, V.M.; Bier, E.; Akbari, O.S. Assessment of a split homing based gene drive for efficient knockout of multiple genes. G3: Genes, Genomes, Genetics https://doi.org/10.1534/g3.119.400985 (2019)
Grunwald, H.A.; Gantz, V.M.; Poplawski, G.; Xu, X.-R. S.; Bier, E.; Cooper, K.L. Super-Mendelian inheritance mediated by CRISPR/Cas9 in the female mouse germline. Nature 565, 105-109 (2019)
Guichard, A.; Haque, T.; Bobik, M.; Xu, X.-R.S.; Klanseck, C.; Kushwah, R.B.S.; Berni, M.; Kaduskar, B.; Gantz, V.; Bier, E. Efficient allelic-drive in Drosophila. Nature Communications 10, 1640 (2019)
Chida, A.R.; Ravi, S.; Jayaprasad, S.; Paul, K.; Saha, J.; Suresh, C.; Whadgar, S.; Kumar, N.; K. Rao, R.; Ghosh, C.; Choudhary, B.; Subramani, S.; Srinivasan, S. A near-chromosome level genome assembly of Anopheles stephensi. bioRxiv 2020.04.27.063040; doi: https://doi.org/10.1101/2020.04.27.063040